Single Replacement Single replacement reactions consist of atoms of an element replacing atoms of a second less reactive element in a compound. 2na 2hoh h 2 2naoh 8.
Examples Of Single Replacement Reactions Science Struck
In this general reaction element A is a metal and replaces element B also a metal in the compound.

. Explanation of single-replacement reaction. The other reactant will be a compound. Cu ai 2 so 4 nr 11.
A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. A and B must either both be metals where C is an anion or they must both be halogens where C is a cation. Fe pbno 3 2 pb feno 3 2 9.
The atom is either a metal or nonmetal. A metal replaces another metal that is in solution. McGraw-Hill Dictionary of Scientific Technical Terms 6E Copyright 2003 by.
Estimated5 minsto complete Progress Practice Single-Replacement Reactions Practice Add to Library Details Resources Download Quick Tips NotesHighlights Vocabulary Replacement Reaction Loading. A BC B. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound.
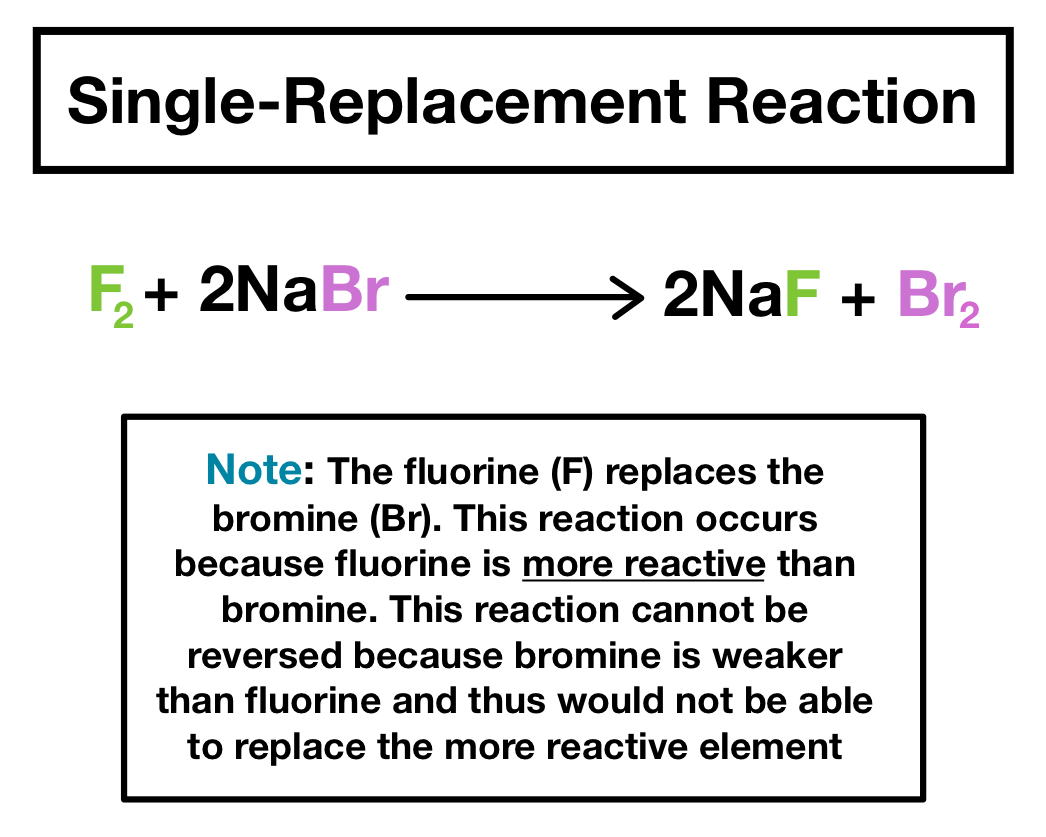
Double-Replacement Reactions When components of two ionic compounds are swapped two new compounds are formed. Chlorine however replaces only bromide iodide and astatide ions and bromine only iodide and astatide ions. Single displacement reaction calculator M 1 X M 2 Cs 1 X 1- Cs Cs X Cs No Reaction Where X1- is a monovalent anion A single replacement reaction aka single displacement reaction will occur if M 1 cation is less reactive than M 2.
The general form of a single-replacement also called single-displacement reaction is. Single replacement reaction definition chemistry. There are two types of single replacement reactions.
A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound. Single replacement reactions or single displacement reactions involve the replacement of an atom or an ion from one compound by a more reactive compound. Single-Replacement Reactions Chemical reaction involving ions where one element is replaced by another in a compound.
Cu h 2 o nr 10. ABC B AC. Typically you will be given the left-hand reactant side and asked to provide the products to the reaction.
Single Replacement Reactions occur with one atom with no charge and one compound. Fluorine replaces any other halide ion from its compounds as shown in the following equations. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
Zn 2agno 3 2ag znno 3 2 3. Single Replacement Reaction In a single replacement or single displacement reaction one uncombined element replaces another in a compound or trades places with it. 2al 3pbno 3 2 3pb 2alno.
A single-displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant. Chemistry single replacement reaction worksheet answer key 1. Find out information about single-replacement reaction.
In single replacement one reactant is always an element. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. 1172 A BC AC B.
It does not matter if the element is written first or second on the reactant side. A chemical reaction in which an element replaces one element in a compound. Single displacement reactions take the form.
Single-Replacement Reactions A single replacement reaction also known as a single displacement reaction occurs when one element in a molecule is swapped for another. This type of a reaction can be depicted in the following manner. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
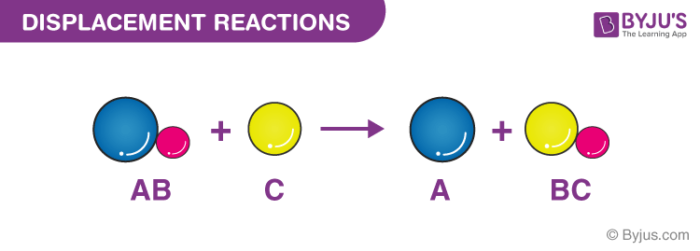
In these reactions a free element reacts with a compound to form another compound and release one of the elements of the original compound in the elemental stateThere are two different possibilities. A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound. This can be modeled by the equation A BC AC B.
REACTION GUIDELINES REACTION FORMAT REACTION DESCRIPTION REACTION CATEGORY. One cation ion replaces another. Looking for single-replacement reaction.
ZnCuCl2 Cu ZnCl2. A BC AC B Double Replacement Reaction. A B-C B A-C.
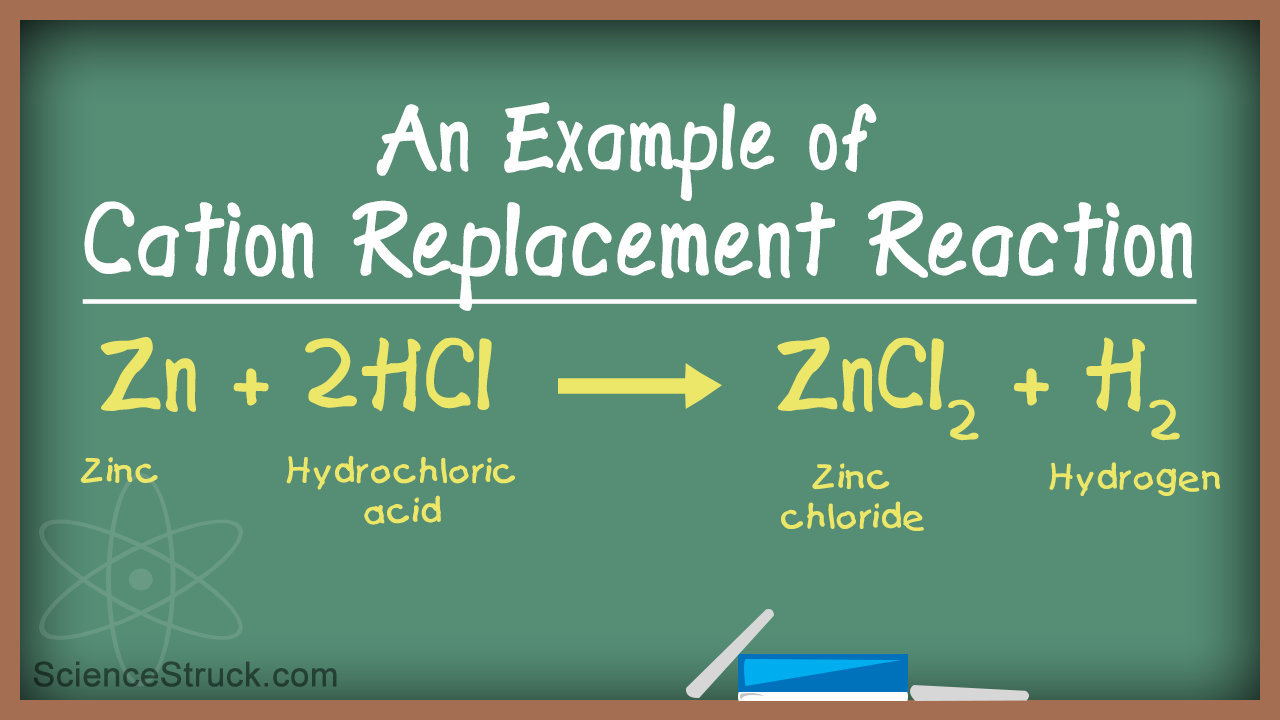
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. Cu feso 4 nr 7. For example 2 HClaq Zns ZnCl 2 aq H 2 g.
Ag kno 3 nr 2. 2li 2hoh h 2 2lioh 6. A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products.
It is also known as a single-replacement reaction. A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products. 2al 3h 2 so 4 3 h 2 al 2 so 4 3 4.
Neutralization precipitation and gas formation are types of double. Other articles where replacement reaction is discussed. The reactivity order corresponds to the reactivity series of the metals.
Free fluorine chlorine bromine and iodine are expected to replace astatide ions. 2 K 2 H 2 O 2 K O H H. Cl 2 2 ki i 2 2kcl 5.
One anion - ion replaces another. The general form of a single replacement reaction is.

Double Replacement Reaction Definition And Examples

Single Replacement Reaction Definition And Examples

Single Replacement Single Displacement Reaction
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
Types Of Chemical Reactions With Examples
Displacement Reactions Definition Types Single Double Examples
Single Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
0 comments
Post a Comment